Microbial Minds and Machine Models: Harnessing Artificial Intelligence to Revolutionize Microbiological Research
Keywords:
Artificial intelligence, Machine learning, Microbiology, Deep learning, Bioinformatics, Metagenomics, Predictive modelingAbstract
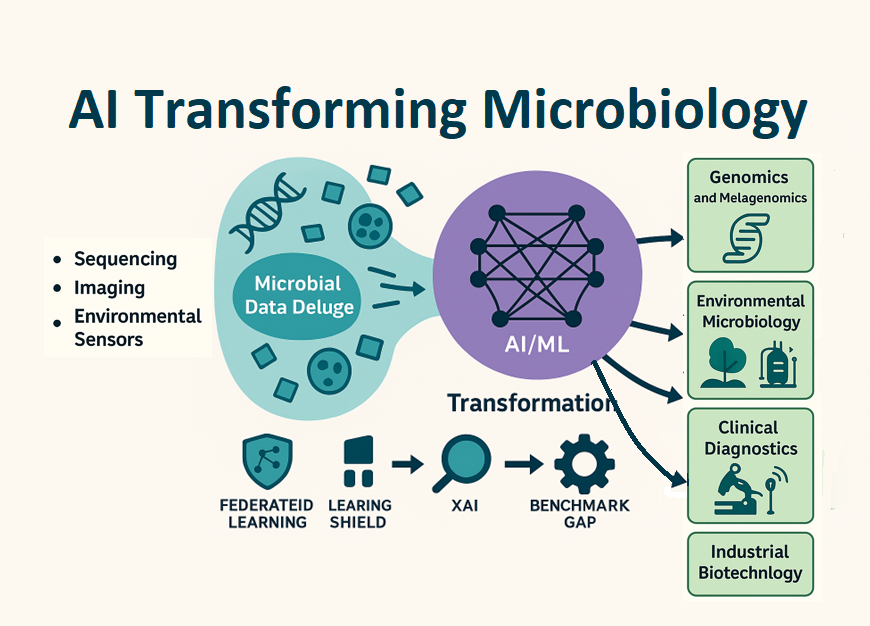
The rapid growth of microbiological data driven by high-throughput sequencing, automated imaging, and environmental sensors has exceeded the capabilities of traditional analytical methods. Artificial intelligence (AI), especially machine learning (ML) and deep learning (DL), has become a transformative tool for finding meaningful patterns in these complex, high-dimensional datasets. This review critically explores how AI is integrated into key microbiological areas, including microbial genomics and metagenomics, environmental microbiology, clinical diagnostics, and industrial biotechnology.
We examine how AI models have sped up genome annotation, enabled detailed phenotypic profiling, enhanced pathogen detection, and optimized bioprocess control. Notable examples include convolutional neural networks for classifying microbial colonies, transformer-based architectures for predicting antibiotic resistance, and generative models for designing synthetic biology applications. However, significant challenges still exist, such as data sparsity, limited interpretability, and the lack of benchmarking in ecological and clinical settings.
We highlight emerging solutions like explainable AI, federated learning, and hybrid mechanistic data-driven models. These approaches aim to improve transparency, generalizability, and responsible use in microbiological settings. By pinpointing current limitations and outlining future directions, this review seeks to guide researchers, clinicians, and bioengineers in using AI tools to advance microbiological discovery and innovation. It is the first review to combine AI applications across clinical, environmental, and industrial microbiology while addressing ethical and infrastructural challenges, targeting interdisciplinary researchers, clinicians, and bioengineers interested in implementing AI-driven solutions.
Downloads
References
References
1. Jumper, J., et al., Highly accurate protein structure prediction with AlphaFold. nature, 2021. 596(7873): p. 583-589.
2. Arango-Argoty, G., et al., DeepARG: a deep learning approach for predicting antibiotic resistance genes from metagenomic data. Microbiome, 2018. 6(1): p. 23.
3. Alsulimani, A., et al., The impact of artificial intelligence on microbial diagnosis. Microorganisms, 2024. 12(6): p. 1051.
4. Lin, Z., et al., Evolutionary-scale prediction of atomic-level protein structure with a language model. Science, 2023. 379(6637): p. 1123-1130.
5. Zhang, K., et al., A fast, scalable and versatile tool for analysis of single-cell omics data. Nature methods, 2024. 21(2): p. 217-227.
6. Steen, A.D., et al., High proportions of bacteria and archaea across most biomes remain uncultured. The ISME journal, 2019. 13(12): p. 3126-3130.
7. Topçuoğlu, B.D., et al., A framework for effective application of machine learning to microbiome-based classification problems. MBio, 2020. 11(3): p. 10.1128/mbio. 00434-20.
8. Chau, K.D., et al., Annual variation across functional traits: The effects of precipitation and land use on four wild bee species. Ecological Entomology, 2025.
9. König, S., et al., TaqMan real-time PCR assays to assess arbuscular mycorrhizal responses to field manipulation of grassland biodiversity: effects of soil characteristics, plant species richness, and functional traits. Applied and Environmental Microbiology, 2010. 76(12): p. 3765-3775.
10. Mian, S.M., et al. Artificial intelligence (AI), machine learning (ML) & deep learning (DL): A comprehensive overview on techniques, applications and research directions. in 2024 2nd International Conference on Sustainable Computing and Smart Systems (ICSCSS). 2024. IEEE.
11. Bolyen, E., et al., Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nature biotechnology, 2019. 37(8): p. 852-857.
12. Oh, M. and L. Zhang, Deepbiogen: Generalizing predictions to unseen sequencing profiles via visual data augmentation. bioRxiv, 2021: p. 2021.05. 06.443027.
13. Ditzler, G., R. Polikar, and G. Rosen, Multi-layer and recursive neural networks for metagenomic classification. IEEE transactions on nanobioscience, 2015. 14(6): p. 608-616.
14. Allen, A., et al., A racially unbiased, machine learning approach to prediction of mortality: algorithm development study. JMIR public health and surveillance, 2020. 6(4): p. e22400.
15. Pasolli, E., et al., Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell, 2019. 176(3): p. 649-662. e20.
16. Pascoal, F., et al., Definition of the microbial rare biosphere through unsupervised machine learning. Communications Biology, 2025. 8(1): p. 544.
17. Tjoa, E. and C. Guan, A survey on explainable artificial intelligence (xai): Toward medical xai. IEEE transactions on neural networks and learning systems, 2020. 32(11): p. 4793-4813.
18. Sczyrba, A., et al., Critical assessment of metagenome interpretation—a benchmark of metagenomics software. Nature methods, 2017. 14(11): p. 1063-1071.
19. Liang, Q., et al., DeepMicrobes: taxonomic classification for metagenomics with deep learning. NAR Genomics and Bioinformatics, 2020. 2(1): p. lqaa009.
20. Dukovski, I., et al., A metabolic modeling platform for the computation of microbial ecosystems in time and space (COMETS). Nature protocols, 2021. 16(11): p. 5030-5082.
21. Halder, A.K., et al., Machine learning-based prediction of acquired antimicrobial resistance in multiple bacterial species using K-mer analysis, mutation detection, and AMR gene profiling. 2025, Brac University.
22. Pearl, J., The seven tools of causal inference, with reflections on machine learning. Communications of the ACM, 2019. 62(3): p. 54-60.
23. Wood, D.E., J. Lu, and B. Langmead, Improved metagenomic analysis with Kraken 2. Genome biology, 2019. 20(1): p. 257.
24. Nissen, J.N., et al., Improved metagenome binning and assembly using deep variational autoencoders. Nature biotechnology, 2021. 39(5): p. 555-560.
25. Rives, A., et al., Biological structure and function emerge from scaling unsupervised learning to 250 million protein sequences. Proceedings of the National Academy of Sciences, 2021. 118(15): p. e2016239118.
26. Cordier, T., et al., Embracing environmental genomics and machine learning for routine biomonitoring. Trends in microbiology, 2019. 27(5): p. 387-397.
27. Tripathi, B.M., et al., Soil pH mediates the balance between stochastic and deterministic assembly of bacteria. The ISME journal, 2018. 12(4): p. 1072-1083.
28. Gerhard, W.A. and C.K. Gunsch, Microbiome composition and implications for ballast water classification using machine learning. Science of the Total Environment, 2019. 691: p. 810-818.
29. Jiang, J., et al., Machine learning to predict dynamic changes of pathogenic Vibrio spp. abundance on microplastics in marine environment. Environmental Pollution, 2022. 305: p. 119257.
30. Gay, B.A., et al., Investigating permafrost carbon dynamics in Alaska with artificial intelligence. Environmental Research Letters, 2023. 18(12): p. 125001.
31. Tripathi, A., et al., The gut–liver axis and the intersection with the microbiome. Nature reviews Gastroenterology & hepatology, 2018. 15(7): p. 397-411.
32. Kim, H., et al., Deep learning frameworks for rapid gram stain image data interpretation: protocol for a retrospective data analysis. JMIR Research Protocols, 2020. 9(7): p. e16843.
33. Ahsan, M.A., et al., DeepPathway: Predicting Pathway Expression from Histopathology Images. bioRxiv, 2025: p. 2025.07. 21.665956.
34. Nguyen, M., et al., Using machine learning to predict antimicrobial MICs and associated genomic features for nontyphoidal Salmonella. Journal of clinical microbiology, 2019. 57(2): p. 10.1128/jcm. 01260-18.
35. Dong, Y., et al., TGC-ARG: Anticipating Antibiotic Resistance via Transformer-Based Modeling and Contrastive Learning. International Journal of Molecular Sciences, 2024. 25(13): p. 7228.
36. Zhang, J., et al., Large language model for horizontal transfer of resistance gene: From resistance gene prevalence detection to plasmid conjugation rate evaluation. Science of The Total Environment, 2024. 931: p. 172466.
37. Si, Y., et al., Enhancing clinical concept extraction with contextual embeddings. Journal of the American Medical Informatics Association, 2019. 26(11): p. 1297-1304.
38. Avershina, E., et al., AMR-Diag: Neural network based genotype-to-phenotype prediction of resistance towards β-lactams in Escherichia coli and Klebsiella pneumoniae. Computational and Structural Biotechnology Journal, 2021. 19: p. 1896-1906.
39. Bhargava, A., et al., FDA-authorized AI/ML tool for sepsis prediction: development and validation. NEJM AI, 2024. 1(12): p. AIoa2400867.
40. Petsagkourakis, P., et al., Reinforcement learning for batch-to-batch bioprocess optimisation, in Computer Aided Chemical Engineering. 2019, Elsevier. p. 919-924.
41. Bordbar, A., et al., Constraint-based models predict metabolic and associated cellular functions. Nature Reviews Genetics, 2014. 15(2): p. 107-120.
42. Yang, K.K., Z. Wu, and F.H. Arnold, Machine-learning-guided directed evolution for protein engineering. Nature methods, 2019. 16(8): p. 687-694.
43. Camacho, D.M., et al., Next-generation machine learning for biological networks. Cell, 2018. 173(7): p. 1581-1592.
44. Koch, M., T. Duigou, and J.-L. Faulon, Reinforcement learning for bioretrosynthesis. ACS synthetic biology, 2019. 9(1): p. 157-168.
45. Wang, X.-W., T. Wang, and Y.-Y. Liu, Artificial Intelligence for Microbiology and Microbiome Research. arXiv preprint arXiv:2411.01098, 2024.
46. Roy, G., et al., Deep learning methods in metagenomics: a review. Microbial genomics, 2024. 10(4): p. 001231.
47. Novielli, P., Artificial Intelligence for the Study of Agri-food Systems. 2025.
48. Holzinger, A., et al., What do we need to build explainable AI systems for the medical domain? arXiv preprint arXiv:1712.09923, 2017.
49. Nguyen, Q.H., et al., eMIC-AntiKP: estimating minimum inhibitory concentrations of antibiotics towards Klebsiella pneumoniae using deep learning. Computational and Structural Biotechnology Journal, 2023. 21: p. 751-757.
50. McCall, A. and A. Mccall, AI for Scientific Discovery: Automating Hypothesis Generation. 2025.
51. Chen, G. and J. Shen, Artificial intelligence enhances studies on inflammatory bowel disease. Frontiers in Bioengineering and Biotechnology, 2021. 9: p. 635764.
52. Kovari, A., AI for decision support: Balancing accuracy, transparency, and trust across sectors. Information, 2024. 15(11): p. 725.
53. Science, C., et al., AI-Enabled Biological Design and the Risks of Synthetic Biology, in The Age of AI in the Life Sciences: Benefits and Biosecurity Considerations. 2025, National Academies Press (US).
Downloads
Published
Submitted
Revised
Accepted
Issue
Section
License
Copyright (c) 2025 Zarrindokht Emami; Anahita Jenab, Kouroush Jenab (Author)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.