Optimized Production Astaxanthin on Sugarcane molasses by X. dendrorhous in 3-LBioreactor
Keywords:
Antioxidant, Astaxanthin, Carotenoid, Fermaentation, Molasses, Response Surface Methodology, Xanthophyllomyces dendrorhousAbstract
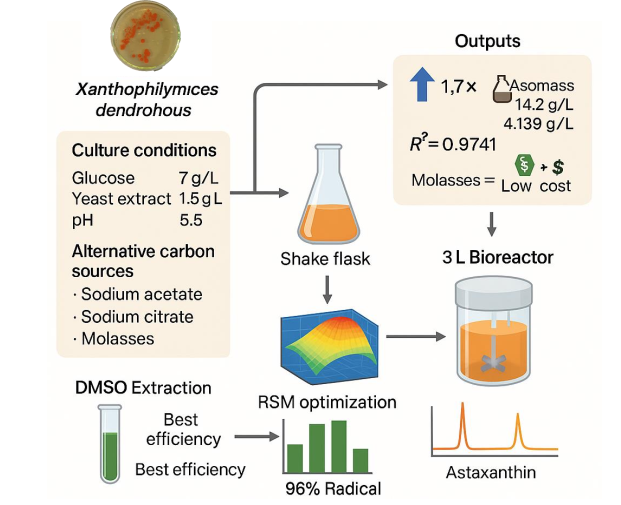
Astaxanthin, a high-value xanthophyll carotenoid with potent antioxidant properties, has garnered significant interest in nutraceutical and pharmaceutical industries. Xanthophyllomyces dendrorhous is a promising microbial source for astaxanthin biosynthesis; however, its industrial application is often constrained by suboptimal yields and high production costs. In this study, central composite design with 20 run was used to optimize process parameters of glucose concentration (7–13 g/L), yeast extract (1.5–4.5 g/L), and initial pH (5.5–7.5) in shake flask cultures. The optimized conditions (7 g/L glucose, 1.5 g/L yeast extract, pH 5.5) led to a 1.7-fold increase in astaxanthin production, with maximum biomass yields of 14.20 g/L (wet) and 4.139 g/L (dry). The regression model showed high predictive accuracy (R² = 0.9741; F = 41.78). To further reduce production costs, alternative carbon sources, including sodium acetate, sodium citrate, and sugarcane molasses (10 g/L), were evaluated. sugarcane molasses supported pigment yields comparable to glucose-based media while substantially lowering media costs. Among four extraction methods tested, DMSO-based extraction demonstrated the highest recovery efficiency. The extracted pigment exhibited 96% DPPH radical scavenging activity, indicating strong antioxidant potential. Results were validated in a 3-L bioreactor (at 22–25 °C, 120 rpm, 4 L/min aeration, and initial pH 5.5), confirming process scalability. These findings demonstrate that integrating medium optimization and low-cost substrates represents a viable and scalable strategy for enhanced microbial astaxanthin production in biotechnological applications.
Downloads
References
1. Maoka T. Carotenoids as natural functional pigments. Journal of Natural Medicines. 2020;74:1-16.
2. Ambati RR, et al. A review on biological activities of astaxanthin and its potential use in human health. Marine Drugs. 2014;12(1):128-52.
3. Fakhri S, et al. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacological Research. 2018;136:1-20.
4. Guerin M, et al. Haematococcus astaxanthin: applications for human health and nutrition. Trends in Biotechnology. 2003;21(5):210-6.
5. Fassett RG, Coombes JS. Astaxanthin in cardiovascular health and disease. Molecules. 2012;17(2):2030-48.
6. Capelli B, et al. Synthetic astaxanthin is significantly inferior to algal-based: A review. Nutrafoods. 2013;12:145-52.
7. Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea FM. Astaxanthin: a review of its chemistry and applications. Critical reviews in food science and nutrition. 2006;46(2):185-96.
8. Lorenz RT, Cysewski GR. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends in biotechnology. 2000;18(4):160-7.
9. Mata-Gómez LC, et al. Biotechnological production of carotenoids by yeasts: An overview. Microbial Cell Factories. 2014;13:12.
10. Bhatt PC, Ahmad M, Panda BP. Enhanced bioaccumulation of astaxanthin in Phaffia rhodozyma by utilising low-cost agro products as fermentation substrate. Biocatalysis and Agricultural Biotechnology. 2013;2(1):58-63.
11. Yamano Y, et al. Metabolic engineering of X. dendrorhous for high astaxanthin production. Applied Microbiology and Biotechnology. 2020;104:6073-83.
12. Rodríguez-Sáiz M, de la Fuente JL, Barredo JL. Xanthophyllomyces dendrorhous for the industrial production of astaxanthin. Applied microbiology and biotechnology. 2010;88(3):645-58.
13. Bhosale P, Gadre RV. Optimization of carotenoid production by Rhodotorula glutinis using RSM. Biotechnology Letters. 2001;23(23):1791-5.
14. Niu T, et al. Comparative evaluation of astaxanthin extraction methods from Haematococcus pluvialis. Algal Research. 2017;23:122-7.
15. Raja R, et al. A comprehensive review on extraction techniques and applications of natural carotenoids. Journal of Food Science and Technology. 2021;58:3296-312.
16. Sowmya R, Sachindra NM. Evaluation of antioxidant activity of carotenoid extract from shrimp processing byproducts by in vitro assays and in membrane model system. Food Chemistry. 2012;134(1):308-14.
17. Harith ZT, de Andrade Lima M, Charalampopoulos D, Chatzifragkou A. Optimised production and extraction of astaxanthin from the yeast Xanthophyllomyces dendrorhous. Microorganisms. 2020;8(3):430.
18. Torres-Haro A, Gschaedler A, Mateos-Diaz JC, Herrera-Lopez EJ, Camacho-Ruiz RM, Arellano-Plaza M. Improvement of a specific culture medium based on industrial glucose for carotenoid production by Xanthophyllomyces dendrorhous. Processes. 2021;9(3):429.
19. Pan X, Li T, Wang B, Qi S, Yang D, Huang Z, et al. Metabolic mechanism of astaxanthin biosynthesis in Xanthophyllomyces dendrorhous in response to sodium citrate treatment. Bioresources and Bioprocessing. 2023;10(1):29.
20. Hu ZC, Zheng YG, Wang Z, Shen YC. pH control strategy in astaxanthin fermentation bioprocess by Xanthophyllomyces dendrorhous. Enzyme and Microbial Technology. 2006;39(4):586-90.
21. Yamamoto K, Hara KY, Morita T, Nishimura A, Sasaki D, Ishii J, et al. Enhancement of astaxanthin production in Xanthophyllomyces dendrorhous by efficient method for the complete deletion of genes. Microbial Cell Factories. 2016;15:1-8.
22. Libkind D, Moliné M, Tognetti C. Isolation and selection of new astaxanthin producing strains of Xanthophyllomyces dendrorhous. Microbial carotenoids From Fungi: Methods and protocols2012. p. 183-94.
23. Urnau L, Colet R, Soares VF, Franceschi E, Valduga E, Steffens C. Extraction of carotenoids from Xanthophyllomyces dendrorhous using ultrasound‐assisted and chemical cell disruption methods. The Canadian Journal of Chemical Engineering. 2018;96(6):1377-81.
24. Wu W, Lu M, Yu L. A new environmentally friendly method for astaxanthin extraction from Xanthophyllomyces dendrorhous. European Food Research and Technology. 2011;232:463-7.
25. Michelon M, de Matos de Borba T, da Silva Rafael R, Burkert CAV, de Medeiros Burkert JF. Extraction of carotenoids from Phaffia rhodozyma: A comparison between different techniques of cell disruption. Food Science and Biotechnology. 2012;21:1-8.
26. Yang L, Qiao X, Liu J, Wu L, Cao Y, Xu J, et al. Preparation, characterization and antioxidant activity of astaxanthin esters with different molecular structures. Journal of the Science of Food and Agriculture. 2021;101(6):2576-83.
Downloads
Published
Submitted
Revised
Accepted
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.